#25-04 Buying Fish is a Can of Worms
In which the E@L finds that pisciphilic parasites are ubiquitous and unpleasant but possibly palatable
An Ugly Surprise
On a recent visit to a local seafood shop I was excited to find fresh swordfish, which is one of my favorite fish, so I purchased two pieces of about a pound each. I pointed out the ones I wanted, and watched the merchant wrap them up in waxed paper. But I made a cardinal mistake: I did not ask if the fish had been previously frozen. And I might not have looked closely enough at the fish.
When I unwrapped the fish in my kitchen, I was surprised to find numerous white worms extending from one of the filets. I was a bit disgusted, but I’ve seen worms before in cod and salmon, so I knew it wasn’t unusual. I tried removing some of them, but there were too many for that approach to be effective. On close examination, I realized that most of them were inactive, ergo dead, so the fish had probably been previously frozen. And most were not entire worms, i.e. they had been cut in half by whomever fileted the fish.
Strangely, the second filet had no worms in it. My first thought was that the wormless filet was the one I had seen in the shop, and perhaps the wormy filet had been lying beneath it. Alternatively, perhaps the worms had worked their way out of the fish between the shop and my home, but that seems unlikely. Nonetheless, the worms were there, so I had to decide what to do with the fish.
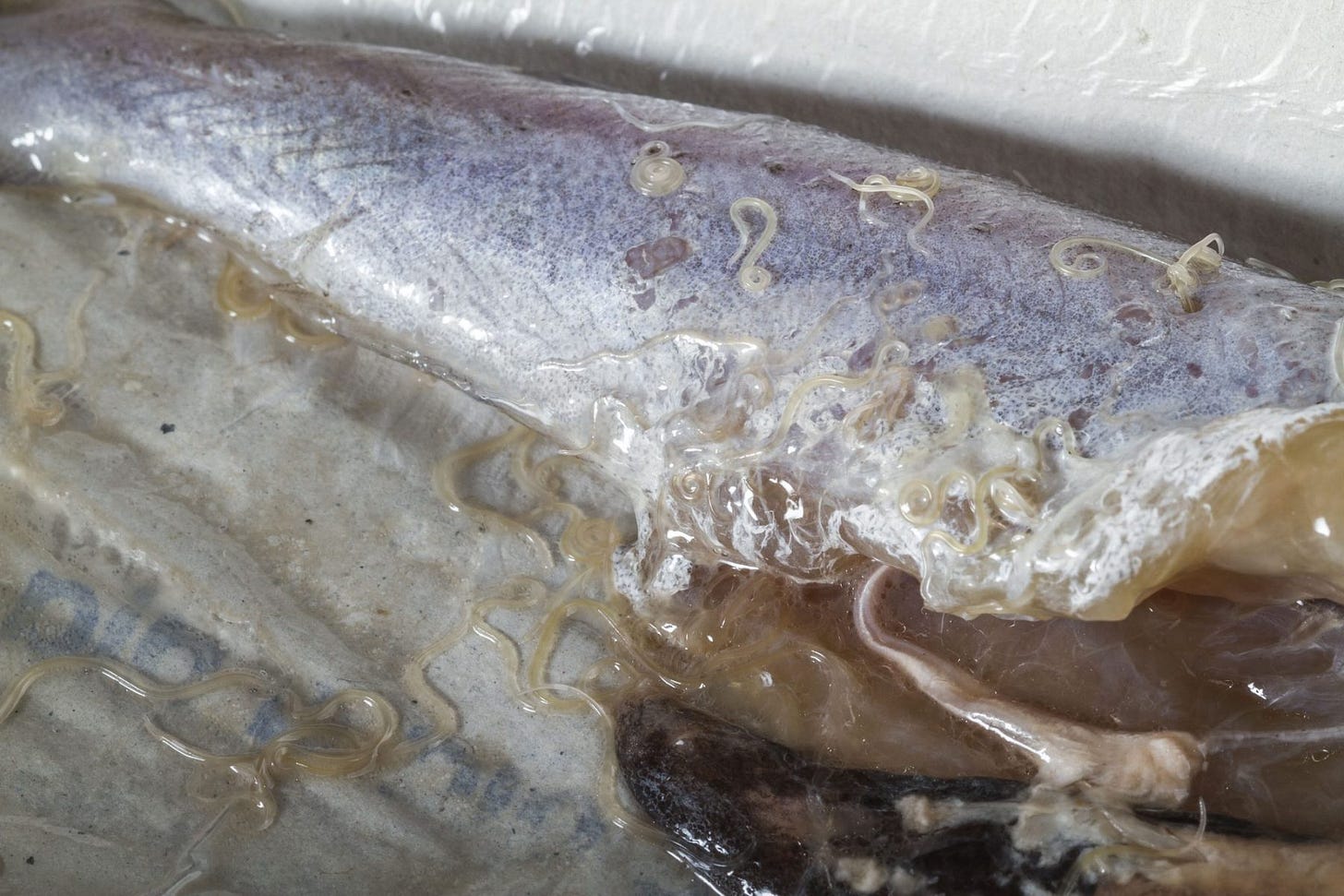
The Host with the Most
Wild fish harbor a great variety of parasites, including roundworms, tapeworms, flukes (parasitic flatworms), protozoans, and copepods, not to mention all of the various harmful bacteria like Listeria and Vibrio. Most of these will never be apparent to us because they are either too small (bacteria), external (copepods), or reside in parts of the fish we don’t eat, such as the liver (roundworms) or gastrointestinal tract (tapeworms). And most are killed by cooking. But roundworms and their larvae live in the flesh so are usually quite obvious to us.
There are many species of marine roundworms, often called “cod worms”, “whale worms”, or “herring worms”, but one of the most common is Anisakis simplex. Other Anisakid genera include Pseudoterranova, Hysterothylacium, and Contracaecum. The life cycle of marine roundworms requires up to three different hosts (which are termed “obligatory”). Adult roundworms live in the guts of marine mammals, including whales, seals, and dolphins. They release eggs that are shed into the water along with mammal feces. The eggs hatch into larvae that are consumed by zooplankton such as krill or copepods, which are then eaten by fish or squid where they develop into the third stage larvae, or L3.
Anisakids are common in Sardines (Sardina pilchardus), Herring (Clupea harengus), Cod (Gadus sp.), European Pollock Pollachius sp, Salmon (Oncorhynchus spp), Hake (Merluccius sp), Chub Mackerel (Scomber japonicus), Pacific Rockfish (Sebastes spp), Swordfish, and many other fishes. If a marine mammal consumes the fish, the parasite can complete its life cycle. If a human eats the fish, though, something else happens.

Conditions in the human gastro-intestinal tract are different from those in marine mammals, so Anisakid parasites cannot thrive there. Most of them die in our GI tract without causing problems. But this may take a week or more, during which they may cause abdominal pain, vomiting, and diarrhea. Not a pleasant thought. If they perforate the wall of the stomach or intestine, they can cause an infection called Anisakiasis, which can lead to severe allergic reaction and anaphylactic shock1. And some worms could survive long enough to work their way up into your respiratory tract and out through your nose. Even less pleasant.
The symptoms of Anisakiasis may be confused with those of gastric ulcers and can only be diagnosed and extracted by endoscopy. This works for the stomach, but not for the small intestine, so parasites there are difficult to detect or remove. Anti-parasitic drugs are not particularly effective against them.
Anisakiasis is an emerging zoonosis of particular concern because of changing dietary habits, especially an increase in consumption of raw fish. In Japan, where sushi is eaten extensively, over 2000 cases of Anisakiasis occur each year, about 90% of the world total. Only about 500 cases are reported in Europe annually, although risk models predict that 10,000 cases should occur2. It is especially common in Spain and Italy, where consumption of ceviche and pickled anchovies is popular.
Seafood processing workers are especially vulnerable to Anisakiasis-like health problems. Over 35% of people working in fishing, fish farming, or fish processing develop occupational asthma. A South African study found that 8% of fish-industry workers were sensitized to Anisakis, and that they were twice as likely to exhibit symptoms of dermatitis after contact with infested fish3. Those that were sensitive to common inhalant allergens were three times as likely to exhibit asthma-related symptoms.
A Parasitic Ecosystem
Multiple species of worms are often present in fish. In swordfish4, these parasite biomes are often distinctive enough that scientists can determine whether an individual fish came from the Mediterranean, the Equatorial Atlantic, or the Northwest Atlantic Ocean. Wild fish tend to have lots of parasitic worms, because they eat wild foods, whereas cultivated (farmed) fish typically do not, because they are fed with a pre-sterilized pelleted food diet. A study done in Norway 5 found that diversity of all parasites was much higher in wild cod than in farmed cod that were hatched and raised in indoor tanks, then transferred to sea cages where they were fed pelleted diets. In addition, Anisakis simplex was present in 87% of wild cod, but only in 1% of hatchery raised farmed cod. Similarly, Anisakids were absent from rainbow trout Oncorhynchus mykiss in a Danish aquaculture system6, though they were abundant in other species of wild fish caught locally.
A meta-analysis of 123 studies published over a 50 year period (1962-2015), including 55,000 fish specimens of 215 species, and 755 parasite observations, found that density (worms per fish) of Anisakis sp. increased by a factor of 283 during that period, whereas densities of Pseudoterranova sp. did not increase7. More recently, scientists at the University of Washington published a study using a serendipitously discovered trove of canned Alaskan salmon accumulated over a 40-year period by commercial processors8. They found that the density of Anisakid worms in canned salmon increased from 1979 to 2019 in samples of chum (Oncorhynchus keta) and pink salmon (O. gorbuscha), but that there was no change in samples of canned sockeye (O. nerka) or coho salmon (O. kisutch). Reasons for this discrepancy may be due to different feeding or habitat requirements of the salmon species, or perhaps that different species of parasites infest different species of salmon, and they responded differently to changes in environmental conditions.
In an earlier study, however, some of the same authors examined parasites from 699 preserved museum specimens of eight non-salmonid fish species collected from Puget Sound over the last 140 years (1880-2019)9. They found 85 parasite taxa including 14 species of copepods (external), 14 species of Monogenea (external flatworms), 5 Acanthocephalans, 15 Nematoda, 12 Cestoda (tapeworms), and 33 Trematoda (internal flukes). In case you have forgotten your flatworm classification, you can brush up here.
Some types of parasites had declined significantly in that time period. Groups that had declined were primarily those that required three obligate hosts in their life cycle, including Anisakis sp. and other roundworms, as well as most tapeworms and flukes. These groups declined at the rate of about 11% per decade. This rate of decline is similar to those for birds (6%), terrestrial vertebrates (7%), and terrestrial insects (9%) over the same time period.
Ten parasite species had completely disappeared in the last 40 years, and nine of those required three or more hosts. In contrast, groups that had not declined were those that required only one or two obligate hosts in their life cycle, e.g. copepods and monogeneans. After examining many associated factors, including fish size, age, time, and location, the authors concluded that declines in abundance were significantly associated with increasing water temperature. Abundance of 3-host parasites declined by 38% for every 1° C increase in sea surface temperature.
But how would temperature affect the abundance of parasites? It could be simply an index of overall climate change. Other concomitant changes in pH, dissolved oxygen, or harmful algal blooms could play a role, but long-term records for those parameters don’t exist. Direct effects of temperature on parasite physiology, e.g. increasing reproduction, are unlikely, since host bodies tend to buffer the parasites from environmental insults.
Increased ocean temperatures could speed up the life cycle of the parasites, increase fish growth rates, and possibly predation intensity by fish. However, this does not account for the declines in parasite populations in Puget Sound fish. That may simply be due to the mix of parasites and conditions found there.
The Rube Goldberg of the Animal World
To gain some insight into this subject, I contacted Dr. Chelsea Wood at the University of Washington, who was the principal investigator on two of these studies. She told me that it was very difficult to identify to species the individual parasites in canned or preserved fish, and that different species of parasites, even within the same family, could respond very differently to environmental changes.
It helps to understand the life cycle of multi-host parasites. To complete their life cycle, they need to find a series of three or more different hosts, all at the right time, as the parasite morphs from one larval stage to another. Dr. Wood likened this process to a Rube Goldberg machine. For those few of you who don’t know, Rube Goldberg was an artist who devised overly complicated machines to complete simple tasks, such as watering a plant, using a series of impractical connections involving clocks, mousetraps, rolling balls, falling buckets, levers, etc. If even one of those devices failed to work, the whole machine would fail.
Now imagine that you are a parasite, and to survive you need to find a specific animal host, or be consumed by it, at a specific time in your life, that may be only a brief interval. And that you must do this multiple times before you reach your final destination host. This results in a “winner-loser” scenario; those parasites that find the correct hosts each time win. If one or more hosts are missing from the equation, perhaps due to climate change, you lose. This creates a bottleneck in the parasite life cycle.
Another possibility is a change in phenology, that is, the seasonal occurrence of life cycle events. If climate change causes the host (or parasite) to change the timing of its feeding, mating, or migration, the parasite-host connection may become mis-aligned, creating a potential failure point.
Dr. Wood suggested that the increase in prevalence of Anisakid parasites in Alaskan salmon may be due to an increase in the abundance of marine mammals, the final hosts for the parasites. Although some mammal populations are still depressed, such as belugas and Steller sea lions, many species of marine mammals, especially humpback whales, fur seals, and Orcas, have increased since the cessation of large-scale commercial whaling and the implementation of the Marine Mammal Protection Act of 1972. Therefore, increases in parasite prevalence may indicate that marine ecosystems are recovering and rebalancing. But as parasite burdens increase, it may increase the susceptibility of their hosts to disease and mortality as well.
In the end, Dr. Wood said, finding parasites in your fish is really a sign that the fish is healthy, and came from a healthy ecosystem. But that’s not much consolation when you are looking at worms in your seafood.
A Diet of Worms
Nobody wants to eat fish with worms in it. Fortunately, parasites are examined and removed or destroyed at many stages in the fish processing supply chain, and most sushi chefs check for these as well. Encounters with nematode parasites can be minimized by careful handling of fish. If you suspect parasites may be present, slice the fish thinly and hold it up to a bright light, a process called “candling”. You may see small dark coils, where the parasites or their larvae are encased in a tough outer sheath. Then you can simply pick them out with a knife or a pair of forceps.
The worms will be killed if the fish is frozen below -20° C (-4° F) for 60 hours, which is typical in commercial processing, or if it is cooked above 60° C (140° F) for one minute. Most home freezers don’t chill below 0° F, though, and cooking to 140° F will dry out your fish, which is usually best at about 125° F (see Simple Steps to Savory Seafood). So, if you know your fish has been commercially deep-frozen, it is probably fine. And if not, then you either overcook your fish or take a calculated risk.
Pickling and cold-smoking of fish (like gravlax) do not kill the parasites. Likewise, live parasites may persist in ceviche because they are not killed by the combination of onions, hot peppers, and citrus juices. Canned fish, though, such as salmon or tuna, is safe to eat because any roundworms have been killed by the cooking process. Fish used for sushi has usually been frozen deeply enough that parasites should not be a concern. Nonetheless, I make it a point not to eat salmon sashimi because I know the worms could be present.
Another way to eliminate encounters with roundworms is to eat mostly farmed fish, such as salmon or tilapia. This has its own trade-offs, though, in terms of other environmental impacts of fish farming, namely water pollution, external parasite (“sea lice”) infestations, escapement and interbreeding with wild fish stocks, and use of forage fish in feed, etc, as discussed in a previous post.
A Fishy Solution
So, what kind of parasite was in my fish? According to Dr. Wood, the worms in my fish were probably not Anisakis, but more likely a type of tapeworm, whose final host was possibly a shark. And conditions in my body are so different from a shark that they probably wouldn’t have survived.
And what did I do with my fish? I marinated it overnight in a mixture of miso and ginger, then cooked it on my gas grill. Or rather, I overcooked it to a temperature of about 145° F. It was a little dry, but still extremely tasty. And after cooking, I could not identify a single worm. They might even have added a little extra umami flavor to my fish.
Bon Apetit! Itadakimasu!
This issue of Ecologist at Large is available to all readers. However, if you would like to support my work with a one-off contribution, click “Buy me a coffee” below.
Sources
1. Aibinu, I. E., P. M. Smooker, and A. L. Lopata. 2019. Anisakis Nematodes in Fish and Shellfish- from infection to allergies. International Journal for Parasitology: Parasites and Wildlife, 9: 384-393. https://doi.org/10.1016/j.ijppaw.2019.04.007 .
2. Adroher-Auroux, F. J., & Benítez-Rodríguez, R. (2020). Anisakiasis and Anisakis: An underdiagnosed emerging disease and its main etiological agents. Research in Veterinary Science, 132, 535–545. https://doi.org/10.1016/j.rvsc.2020.08.003
3. Nieuwenhuizen, N. et al. 2006. Exposure to the fish parasite Anisakis causes allergic airway hyperreactivity and dermatitis. Journal of Allergy and Clinical Immunology. 117 (5): 1098-1105. https://doi.org/10.1016/j.jaci.2005.12.1357
4. Mattiucci, S., et al. 2014. Metazoan parasite infection in the swordfish, Xiphias gladius, from the Mediterranean Sea and comparison with Atlantic populations: implications for its stock characterization. Parasite. 2014;21:35. Epub 2014 Jul 25. PMID: 25057787; PMCID: PMC4109596. https://doi.org/10.1051/parasite/2014036.
5. Heuch, P.A. et al. 2011. Parasite faunas of farmed cod and adjacent wild cod populations in Norway: a comparison. Aquaculture Environment Interactions. Vol. 2: 1–13. https://doi.org/10.3354/aei00027
6. Skov, J. et al. 2009. Nematode infections of maricultured and wild fishes in Danish waters: A comparative study. Aquaculture Vol. 298 (1–2): 24-28. https://doi.org/10.1016/j.aquaculture.2009.09.024
7. Fiorenza, E.A., et al. 2020. It’s a wormy world: Meta-analysis reveals several decades of change in the global abundance of the parasitic nematodes Anisakis spp. And Pseudoterranova spp. in marine fishes and invertebrates. Global Change Biology 26 (5): 2854-2866. https://doi.org/10.1111/gcb.15048
8. Mastick, N., R. Welicky, A. Katla, B. Odegaard, V. Ng, & C.L. Wood. 2024. Opening a can of worms: Archived canned fish fillets reveal 40 years of change in parasite burden for four Alaskan salmon species.Ecology and Evolution,14, e11043. https://doi.org/10.1002/ece3.11043
9. Wood, C.L. et al. 2023. A reconstruction of parasite burden reveals one century of climate-associated parasite decline. Proceedings of the National Academy of Science. e2211903120. https://doi.org/10.1073/pnas.2211903120





Amazing substack so well done.
I had a dwarf tapeworm in Indonesia that lived in my gut for several months and caused only mild symptoms. I caught it from eating sun dried fish.....as far as we could work out.